Passivation is a critical process used across various industries, including aerospace, defense, electronics, and medical, to enhance the corrosion resistance and durability of stainless steel components. This chemical treatment forms a thin, protective oxide layer on the metal surface, which acts as a barrier against environmental degradation. Understanding the science behind passivation, the different techniques involved, and its practical applications can help professionals make informed decisions about maintaining the integrity of their stainless steel parts. In this blog, we will explore key concepts, chemical reactions, benefits, challenges, and safety considerations related to stainless steel passivation. At its core, passivation is a process designed to improve the corrosion resistance of stainless steel by removing free iron particles from the surface and forming a passive oxide layer. Free iron, if left untreated, can lead to rust, oxidation, and discoloration when exposed to moisture or oxygen. By eliminating these contaminants, passivation ensures that the surface remains clean and ready to develop a strong, protective film. Free Iron: Residual iron particles on the surface can act as catalysts for corrosion. Removing them is essential for long-term performance. Passive Film: A thin layer of metal oxide that forms on the surface during passivation, acting as a protective shield against corrosion. Surface Contamination: Dirt, oil, grease, or other foreign substances can interfere with the formation of the passive layer. Thorough cleaning before passivation is crucial. Conversion Coating: A general term for surface treatments that improve corrosion resistance. Passivation is a specific type of conversion coating tailored for stainless steel. The science of passivation involves chemical reactions between the stainless steel surface and the passivating solution. When immersed in a solution like citric acid or nitric acid, the surface reacts to form a dense, protective oxide layer. This layer not only prevents further corrosion but also enhances the material’s overall performance in harsh environments. Understanding the chemistry behind these reactions helps optimize the passivation process, ensuring maximum effectiveness and longevity of the treated parts. There are several methods for passivating stainless steel, each with unique advantages depending on the application. The two most common approaches are citric acid passivation and nitric acid passivation. Citric acid passivation is an eco-friendly alternative to traditional methods, particularly favored in medical and food processing industries. It effectively removes free iron and impurities without the harshness of nitric acid. This method is safer, easier to handle, and meets strict cleanliness standards, making it ideal for sensitive applications. Nitric acid passivation is known for its strong oxidizing properties, making it highly effective at removing contaminants and forming a dense chromium oxide layer. While more aggressive than citric acid, it offers superior corrosion resistance, especially in high-carbon stainless steels or corrosive environments. However, it requires careful handling due to its higher reactivity and potential hazards. Oxidation plays a central role in the formation of the passive layer. Chromium in the stainless steel reacts with oxygen to form a thin layer of chromium oxide. This layer acts as a barrier, preventing direct contact with corrosive agents and significantly improving the material's durability. The passivation process typically includes three main steps: preparation and cleaning, applying the passivation solution, and rinsing and drying. Each stage is vital to ensure the final product has a strong, uniform passive layer. Before passivation, the surface must be thoroughly cleaned to remove oils, dirt, and any residual contaminants. Degreasing and mechanical cleaning are often used to prepare the surface for optimal results. Stainless steel parts are immersed in a passivation solution—either citric or nitric acid—for a specified time. The duration depends on the type of steel, the method used, and the desired outcome. Following ASTM standards is essential for consistency and quality. After immersion, the parts are rinsed to remove any remaining chemicals. Proper drying is important to avoid water spots and ensure the passive layer remains intact. This step completes the passivation process and prepares the parts for use. While passivation is highly beneficial, it can present challenges. Issues such as improper cleaning, incorrect solution concentration, or insufficient immersion time may lead to ineffective passivation. Regular maintenance and adherence to guidelines are necessary to maintain the integrity of the passive layer over time. Safety is a top priority during passivation due to the use of strong acids. Protective gear, proper ventilation, and strict adherence to manufacturer instructions are essential. After passivation, all chemicals should be disposed of responsibly to protect both personnel and the environment. Passivation is a chemical process that removes free iron from the surface of stainless steel, creating a protective oxide layer that improves corrosion resistance and extends the life of the material. Yes, stainless steel benefits from passivation, especially after fabrication or machining. It helps maintain the material’s durability and performance in various environments. Common passivation chemicals include citric acid, nitric acid, and sodium dichromate. These substances help clean the surface and promote the formation of a protective oxide layer. Carbon steel cannot be passivated in the same way as stainless steel. Instead, it requires external coatings or treatments like painting or oiling to prevent corrosion. Factors include the chromium content of the steel, correct immersion time, surface condition, temperature, and solution strength. Each plays a role in ensuring the formation of a strong, uniform passive layer. Shop our Stainless Steel Compression Fittings
Seamless carbon steel pipes are made of steel ingots or solid billets through perforation to make capillaries, which are then hot-rolled, cold-rolled or cold-drawn. The raw material of carbon Steel Pipe is a round tube blank, which is cut by a cutting machine into a billet with a length of about 1 meter, and sent to a furnace for heating through a conveyor belt. The billets are fed into the furnace and heated at about 1200 degrees Celsius. The fuel is hydrogen or acetylene. Furnace temperature control is a critical issue. After the round tube blank is released from the furnace, it is pierced through a pressure punching machine. Generally, the more common punching machine is the conical roller punching machine. This kind of punching machine has high production efficiency, good product quality, large amount of perforation and diameter expansion, and can wear a variety of steel grades. After perforation, the round tube blank is successively cross-rolled, continuously rolled or extruded by three rolls.
Seamless steel pipe is a tubular section or hollow cylinder, usually but not necessarily of circular cross-section, used mainly to convey substances which can flow - liquids and gases (fluids), slurries, powders and masses of small solids.
Hot rolled seamless steel pipe deformation process can be summarized as three stages: extension, perforation and finishing. Hot rolled seamless steel pipes is relative to the cold-rolled, cold rolling is below the recrystallization temperature of the rolling and hot rolled is carried out at above the recrystallization temperature of the rolling. Hot rolled seamless steel pipe can damage the refinement of the crystal grains of the steel, cast microstructure of the steel ingot and eliminate the defects of the microstructure so that the the steel organization compacting, improves the mechanical properties. This improvement is reflected in the rolling direction so that the steel is no longer at a certain extent isotropic; pouring the formation of bubbles, cracks and osteoporosis, under high pressure and temperature can also be welded together.
Hot Rolled Steel Pipes (Seamless Tube) in different size, specifications, grades & thickness as per the client's requirements. The range offered by tubes is available as per the international & national standard and can be available from us at affordable prices. These pipes are used in various application industry like shipbuilding, power plants, oil and gas, automotive, sugar mills and distilleries, cement and construction industries etc.
Carbon Steel Pipes,Mlid Steel Pipe,Seamless Steel Pipe SHANDONG HUITONG STEEL CO.,LTD , https://www.cnmetalsupply.comPassivation of Stainless Steel Parts
Understanding Passivation
Key Terms in Passivation
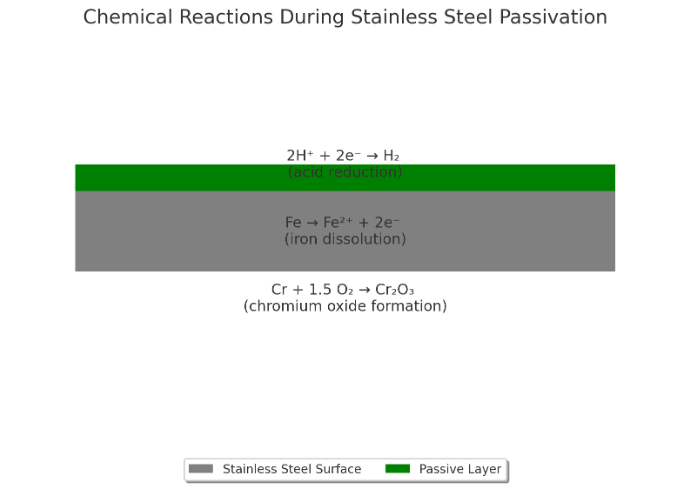
The Science Behind Passivation
Types and Techniques of Passivation
Citric Acid Passivation
Nitric Acid Passivation
The Role of Oxidation in the Passivation Layer
The Procedure of Passivating Stainless Steel
1) Preparation and Cleaning
2) Applying Passivation Chemical
3) Rinsing and Drying

The Challenges of Passivation
Safety Measures During Passivation Process
Key Equipment in Stainless Steel Passivation
 Industrial passivation systems include acid-resistant tanks, temperature-controlled baths, spray rinse stations, and drying ovens. Automated handling systems, such as conveyors and robotic arms, streamline the process. Monitoring tools like pH meters ensure the solution remains effective throughout the operation.
Industrial passivation systems include acid-resistant tanks, temperature-controlled baths, spray rinse stations, and drying ovens. Automated handling systems, such as conveyors and robotic arms, streamline the process. Monitoring tools like pH meters ensure the solution remains effective throughout the operation.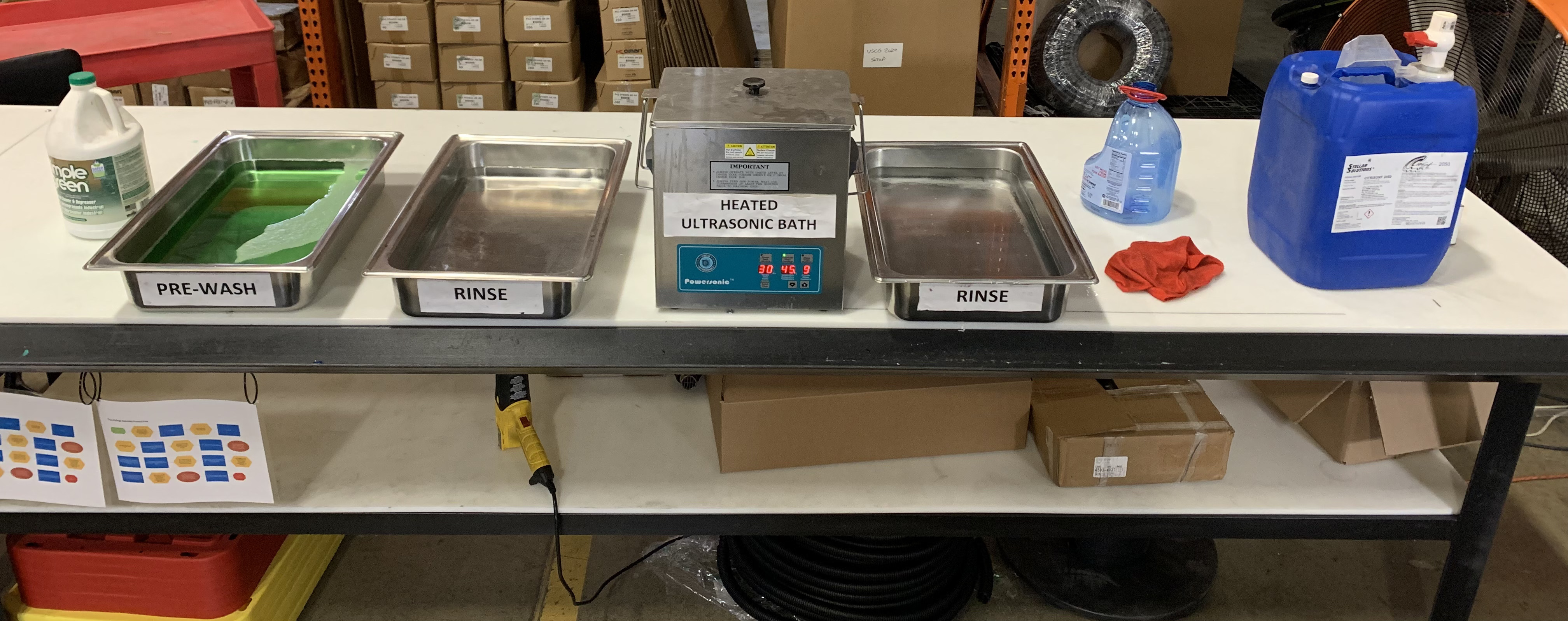
Frequently Asked Questions
What is passivation?
Does stainless steel need passivation?
What chemicals are used in passivation?
Can I passivate carbon steel?
What Factors Influence the Success of the Passivation Process?
Passivation of Stainless Steel Parts
